01 Sep Anatomy of a stressful event: from stress to the immune system – the scientific basis of psychosomatic
Let us start with a concrete example anyone can relate to, from school interrogations to business meetings:
Ask a person to speak in public telling them that they will be evaluated by a panel of behavioral experts, and we will analyze their neurobiological activation.
Probably someone, just by reading this description and thinking that they could be in a similar situation, will have felt a little tension come their way and some of the reactions will be triggered by what we are about to describe, although in a mild form. Probably, just by reading this description the readers of this article will have felt a little tension come their way, and some of their reactions will be triggered by what we are about to describe, although in a mild form. 
The researchers (1) who have analyzed these phenomena in detail agree on the fact that in these cases two mechanisms take place: an easily predictable one and a less obvious one.
1. The first mechanism relates to the activation of the well known reaction of fight or flight, characterized by increased heart rate, blood pressure, cortisol and catecholamines, as well as a number of other processes that we not need to look into. 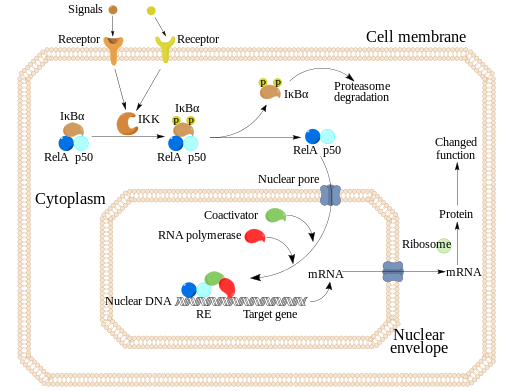
2. The second mechanism activates important inflammatory pathways in the peripheral blood cells, including the activation of the nuclear transcription factor (NF-kB), and leads to a marked increase of pro-inflammatory cytokines in the circulation, including interleukin-6 (IL-6).
Correlations and Connections
But that is not all. There are also significant correlations between this inflammatory response and other factors. For example, subjects exposed to early childhood trauma (in the broad sense of ACEs) show increased inflammatory response to this public-speaking test in comparison to other subjects. Nevertheless, looking in prospective terms, it was observed that people who show a higher inflammatory  response to psychosocial stress have a significantly higher risk of developing depression in the following months (2).
response to psychosocial stress have a significantly higher risk of developing depression in the following months (2).
In addition, inflammation favours the hyper-reactive emotions in general and alters cognitive and motivational processes (3). We have examples of these processes influencing each other negatively, but fortunately there is also a mutual support relationship between emotional and immune responses.
For example, T cells can play a protective role with respect to stress and depression, promoting the proper functioning of cytokines, a change in microglia and neurogenesis in the hippocampus (4). Similarly, cytokines underlying immune responses may have a significant impact when compared with monoamine serotonin, norepinephrine and dopamine at the base of mood regulation (5).
These are only a few examples of how these pathways can create virtuous circles that should now be taken into consideration by any therapeutic approach.  All of these processes also begin to act on an epigenetic level. The inflammation can lead to epigenetic markings able to amplify depressive answers or anxiety in the presence of psychosocial stress.
All of these processes also begin to act on an epigenetic level. The inflammation can lead to epigenetic markings able to amplify depressive answers or anxiety in the presence of psychosocial stress.
Evidence of this bidirectional link between stress and the immune system epigenetic level is provided by the important discovery of how childhood trauma is associated with increased inflammation through epigenetic changes induced by stress in FKBP5, a gene involved in the development of depression and anxiety as well as in sensitivity to gluco-corticoids (6).
We are faced with a situation that poses interesting questions:
- How is it that a stimulus (such as speaking in public) lacking in pathogens activates the immune system?
- How can immune inflammation foster anxiety or depression?
- Is this communication between systems the result of a mistake or something more significant?
- As it is able to change epigenetic expression, is there an adaptive and evolutionary significance behind these mechanisms?
The integrated interpretation between evolutionism and PNEI
 To answer these questions, the evolutionary perspective integrated with the typical systemic reading of PsicoNeuroEndocrinoImmunology might be of great help. If we go back several years, it is clear that the fight or flight system was activated under certain conditions: when survival was at risk because of hunting, being hunted by a predator or adverse environmental conditions.
To answer these questions, the evolutionary perspective integrated with the typical systemic reading of PsicoNeuroEndocrinoImmunology might be of great help. If we go back several years, it is clear that the fight or flight system was activated under certain conditions: when survival was at risk because of hunting, being hunted by a predator or adverse environmental conditions.
In each of these situations human beings were highly likely to be injured, infected, come into contact with the pathogens of the aggressor or harmful environmental substances. This shows that the immune system must necessarily take action together with the fight or flight system.
Evolutionarily, it also makes sense that all systems are as pre-emptive to the perception of a risk as possible in order to prepare the body in advance of reaction. This evolutionary perspective is evident even in neuroscientific and neurobiological research (7), which highlight the central role of the human-pathogen relationship in the development of evolutionary change and adaptation processes.
In addition, bi-directional communications of the central nervous system, stress axis, immune system and digestive system, appear to be the result of a well-articulated evolutionary architecture and can not only be explained by chance (8).
The problem nowadays
These mechanisms are evolutionary or, perhaps, they used to be evolutionary. Today, in fact, the risks that trigger our responses of fight or flight almost never affect physical health, and so do not require the activation of the immune system. Studying for an exam, facing a job interview, getting angry when we are immersed in traffic hardly lead us to encounter pathogens. This has changed, but
the timing of evolutionary processes is slower in our culture
and, thus, the immune response is triggered even if the circumstances do not make it necessary. Another aspect that has changed significantly regards frequency. Do you think that the cavemen’s life conditions were worse than ours? Perhaps they had less comfort, but their stress axis was activated a few times a day, or even a week.
Think about the life of a monkey, how often do you think it is under stress during the day? Now think about the life of an average person in the Western world: they wake up earlier than they would like, face the traffic, look for parking, discuss work issues, sacrifice their passions and social relations in the name of duty or other restrictions, often eating quickly.
life of an average person in the Western world: they wake up earlier than they would like, face the traffic, look for parking, discuss work issues, sacrifice their passions and social relations in the name of duty or other restrictions, often eating quickly.
Of course, our lives are not in danger, but we know that as it tries to identify salient stimuli our amygdala does not differentiate much between real and imagined dangers, and it rightly considers every field invasion field as vital, each thrust to act against their own adaptive advantage, against energy saving and against sociability (9).
What to do? In the light of what we have seen so far it has become necessary to develop an integrated approach that really takes into account all levels of interaction between mind, emotions and the immune system.
We often hear of integration while remaining within the scope of individual disciplines: different psychotherapies integrated with each other, different medications, different body techniques, etc. This mono-theoretical approach does not go very well, but it should be the basis of the formation of every profession. Integration has to take place between different disciplines.
Obviously a psychologist will never perform surgery, and a surgeon will not be able to support a trauma survivor. Yet, all professionals can – and must – act in their area, taking into account all the complexity of the problems they are working on. To give a concrete example of what we have just seen, we can point out that inflammation reduces our ability to respond to rewards and gratifications; moreover, it reduces interest in new information, while increasing sensitivity to adverse stimuli.
In practical terms, it means that it will be difficult to help people “enjoy life” and encourage them to “see their successes” if they are in a state of inflammation. From eating habits to lifestyle and emotional management, from assertive communication to good interpersonal relationships, from the satisfaction of its needs to the ancestral way of thinking, there are many ways to adjust the immune system and bring back the inflammatory mechanisms in physiology.
In contrast to that, this distorted mechanism leads to a higher sensitivity to adverse stimuli and makes people reinforce their beliefs in two main ways: firstly, from a cognitive point of view, it causes people to find negative evidence on which to ruminate; secondly, it reactivates all neurobiological processes of stress and inflammation in the face of every stimulus that reminds them of stressful events. It is therefore important to work on habits, exploiting the neurobiological mechanisms on which evolution relies (detection of clues, routine activations, reducing costs, increasing the pleasure) and not by sheer force of will.
The other system, important to regulate inflammation power, is to activate its link with interoception, a mechanism lying at the basis of our self-regulating capacity.
This can be done in different ways, but the most important one consists in the direct sensory and bodily experience.
References
1.a Pace, T. W. et al. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am. J. Psychiatry 163, 1630–1633 (2006).
1.b Bierhaus, A. et al. A mechanism converting psychosocial stress into mononuclear cell activation. Proc. Natl Acad. Sci. USA 100, 1920–1925 (2003).
2. Aschbacher, K. et al. Maintenance of a positive outlook during acute stress protects against pro-inflammatory reactivity and future depressive symptoms. Brain Behav. Immun. 26, 346–352 (2012).
3. Beauchaine, T. (2001). Vagal tone, development, and Gray’s motivational theory: toward an integrated model of autonomic nervous system func- tioning in psychopathology. Dev. Psychopathol. 13, 183–214. doi: 10.1017/ S0954579401002012
4.a Brachman, R. A., Lehmann, M. L., Maric, D. & Herkenham, M. Lymphocytes from chronically stressed mice confer antidepressant-like effects to naive mice. J. Neurosci. 35, 1530–1538 (2015).
4.b Sandiego, C. M. et al. Imaging robust microglial activation after lipopolysaccharide administration in humans with PET. Proc. Natl Acad. Sci. USA 112, 12468–12473 (2015).
4.c Quan, N. & Banks, W. A. Brain-immune communication pathways. Brain Behav. Immun. 21, 727–735 (2007).
4.d D’Mello, C., Le, T. & Swain, M. G. Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factor-α signaling during peripheral organ inflammation. J. Neurosci. 29, 2089–2102 (2009).
5.a Zhu, C. B. et al. Interleukin-1 receptor activation by systemic lipopolysaccharide induces behavioral despair linked to MAPK regulation of CNS serotonin transporters. Neuropsychopharmacology 35, 2510–2520 (2010).
5.b Neurauter, G. et al. Chronic immune stimulation correlates with reduced phenylalanine turnover. Curr. Drug Metab. 9, 622–627 (2008).
5.c Felger, J. C. et al. Tyrosine metabolism during interferon-α administration: association with fatigue and CSF dopamine concentrations. Brain Behav. Immun. 31, 153–160 (2013).
5.d Maes, M., Leonard, B. E., Myint, A. M., Kubera, M. & Verkerk, R. The new ‘5-HT’ hypothesis of depression: cell-mediated immune activation induces indoleamine 2,3-dioxygenase, which leads to lower plasma tryptophan and an increased synthesis of detrimental tryptophan catabolites (TRYCATs), both of which contribute to the onset of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 35, 702–721 (2011).
5.e Raison, C. L. et al. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-α: relationship to CNS immune responses and depression. Mol. Psychiatry 15, 393–403 (2010).
6. Klengel, T. et al. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat. Neurosci. 16, 33–41 (2013).
7.a Choi, G. B., Dong, H. W., Murphy, A. J., Valenzuela, D. M., Yancopoulos, G. D., Swanson, L. W., and Anderson, D. J. (2005). Lhx6 delineates a path-way mediating innate reproductive behaviors from the amygdala to the hypothalamus. Neuron 46, 647–660.
7.b Swanson, L. W., and Petrovich, G. D. (1998). What is the amygdala? Trends Neurosci. 21, 323–331.
7.c LeDoux, J. E. (2012). Evolution of human emotion: a view through fear. Prog. Brain Res. 195, 431–442. 8.a Bottaccioli F., Psiconeuroendocrinoimmunologia, RED ED.,Milano, (2005).
8.b Mayer, E. A., Knight, R., Mazmanian, S. K., Cryan, J. F. & Tillisch, K. Gut microbes and the brain: paradigm shift in neuroscience. J. Neurosci. 34, 15490–15496 (2014).
8.c Porges, S. W. (1995). Orienting in a defensive world: mammalian modifications of our evolutionary heritage. A Polyvagal Theory. Psychophysiology 32, 301–318. doi: 10.1111/j.1469-8986.1995.tb01213.x 8.d Quan, N. & Banks, W. A. Brain-immune communication pathways. Brain Behav. Immun. 21, 727–735 (2007).
9.a Raison, C. L. & Miller, A. H. The evolutionary significance of depression in Pathogen Host Defense (PATHOS-D). Mol. Psychiatry 18, 15–37 (2013).
9b. Watson, P. J. & Andrews, P. W. Toward a revised evolutionary adaptationist analysis of depression: the social navigation hypothesis. J. Affect. Disord. 72, 1–14 (2002).
9.c Kinney, D. K. & Tanaka, M. An evolutionary hypothesis of depression and its symptoms, adaptive value, and risk factors. J. Nerv. Ment. Dis. 197, 561–567 (2009). 9.d Slavich, G. M. & Irwin, M. R. From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol. Bull. 140, 774–815 (2014).